Innovating Cardiac Care for American Indian Communities: A Conversation with Dr. Sarah De Loizaga

Dr. Sarah de Loizaga
MD

Luke Baldwin
VP of Global Marketing, EchoNous
Luke Baldwin, VP of Global Marketing at EchoNous, recently engaged in a thought-provoking discussion with Dr. Sarah de Loizaga, MD, pediatric cardiologist, advocate for equity in healthcare, and leader in research dedicated to addressing health disparities and advancing access to cardiac care. Read on as they explore how task-shifting and community-centered methods can revolutionize pediatric cardiac screening and provide crucial perspectives on improving healthcare for underserved communities.
Dr. de Loizaga’s journey underscores the importance of community-centered approaches in improving cardiac care access. With her background as a pediatric cardiologist and health equity advocate, she offers practical insights into the effectiveness of task-shifting and collaborative healthcare models for marginalized communities.
[Note: This interview has been edited for clarity and brevity.]
Background and Journey
Luke Baldwin: To start, could you please tell us a little about your background? What do you practice, and how did you get to where you are?
Sarah de Loizaga, MD: I am a pediatric cardiologist at Cincinnati Children’s. Clinically, I work in general cardiology in our outreach clinics. The majority of my time is research-based and focused on addressing health disparities to improve outcomes for people impacted by heart disease.
This has manifested in a few different ways: some work done here locally, such as examining social needs screening for children admitted to the cardiology service, and then, nationally with the American Indian population out in Arizona to improve access to care and early detection of structural heart disease. Then, globally, this work is focused around rheumatic heart disease, mainly out of Uganda – so broadly speaking, addressing health disparities manifesting in a few different ways.
Luke Baldwin: I’m intrigued by that. So, how did health disparities become your primary research focus?
Sarah de Loizaga, MD: It started when I was a pediatric resident here. We get assigned clinics in which we do continuity care. So, you go to that clinic each week. I think that it became evident to me how social needs impact how patients do, how easy it is for them to access care and come to follow-ups.
Sitting in on visits, I realized that despite having a list of things that you think you want to go over in this well-child visit, there are so many social factors that the family is juggling with and trying to address that it’s really low on their list whether their children are brushing their teeth twice a day.
With that in my pediatric training, and then having some mentors who are, I think, leaders in this type of research within general pediatrics that I was lucky enough to work with in my residency, really brought that to the forefront of something that I was interested in trying to look into and incorporate into my career.
So, when I came into fellowship, you know, the requirement is that you do research. At the time, I did not anticipate research being such a significant part of my career. I felt like it would be a checkbox on what I had to do to complete the fellowship. When they asked what my research interest was I shared that I was interested in looking at health disparities and how they impact the kids we care for.within cardiology.
At the time, at least within the subspecialty of cardiology, it wasn’t as much on the radar as it is now or even within other areas of medicine. That has changed, and I think, with COVID especially, it is much more in the forefront of everyone’s mind these days—there is awareness around it, and that it has a significant impact. Some efforts are starting to address that, which is exciting for me, and there’s much more going on.
Health Disparities and Social Determinants of Health
Luke Baldwin: Would you mind explaining what falls under the umbrella of health disparities and how you would define that?
Sarah de Loizaga, MD: So, when I talk about things like health-related social needs, I think a lot of people also think about social determinants of health. It’s the social factors, like access to transportation, food insecurity, factors such as poverty or housing stability—all these things that are, I think, social factors [that] you wouldn’t traditionally think about being related to health, but they do have an impact on health and health outcomes.
So, how a child with asthma does, whether they are living in a home that has dust, debris, insects, and lacks air conditioning in the summer, it’s going to be very different than a child that has asthma and is living in a home that is air-conditioned all the time, where there’s no concern about the electricity being turned off and those sorts of things.
So, it’s these factors around you that do impact how you do with your health and how often you have exacerbations and have to come into the hospital.
Luke Baldwin: As you were talking, I couldn’t help but think that these factors make it hard to stay healthy or get treatment and pose barriers to proper access to care.
Sarah de Loizaga, MD: Yeah. So, those factors also manifest in environmental ways. It’s about where you live and the things in your immediate environment. Those are always the ones that ultimately impact your health.
Origins of the Project: Addressing Disparities in American Indian Healthcare
Luke Baldwin: When did American Indian healthcare and the disparities you identified first come onto your radar?
Sarah de Loizaga, MD: This is a bit of a convoluted story and background. I was working with Dr. Andrea Beaton, who is also part of this work. It was really around rheumatic heart disease, which is her main focus. One of our investigators, Ryan Close, worked with the Indian Health Service in Arizona and some of his work had an infectious disease focus. While he was out there, he did work that looked at Group A strep (Group A Streptococcus) and found that the rates of Group A strep in the tribe that he was working with were 30 times higher than the general population—really high.
We know that Group A strep is the precursor to developing rheumatic heart disease, something we don’t see very much in the US anymore, but something that still impacts many people living in low-and-middle-income countries. He was presenting at a conference and was talking about his work, and someone said, “Have you talked to Andrea Beaton? Because with the high rates of Group A strep, there’s probably also more rheumatic heart disease in the population.” So that’s how we got connected initially.
We were initially going to collaborate with Ryan and look at the prevalence of rheumatic heart disease in the population there. You know, historically, studies that have looked at this have found that there are much higher rates of rheumatic heart disease in indigenous populations, but most of that work needs to be updated, like from the 1990s. Initially, we figured we would do a prevalence study and determine how much rheumatic heart disease there is currently.
That project blossomed because the more we talked to Ryan about this project and developing it, the more it became apparent that beyond just trying to figure out the prevalence of rheumatic heart disease, there was this massive need for having better access to echocardiography and diagnosis for heart disease.
So, we talked to him, and he said, “You know, I order an echo for a patient I may see in my outpatient clinic, and if I’m lucky if it actually gets done, which often it doesn’t, I get that report in three, maybe four months,” which was mind boggling to us because here, you know, you order an echo and it takes maybe a couple of weeks max, but often happens the day of the appointment.
So, there’s a huge barrier for many of these patients when echocardiograms are ordered. Many of these patients have to travel up to four hours to get it, and this is in a community where maybe one in four families has access to a car. So, again, that was a huge barrier, and as you can imagine, this often ends up resulting in a late diagnosis with more significant complications. When you get diagnosed in the late stages, it’s likely due to coming into the hospital when symptoms start, which is not the ideal time to capture that.
Ryan shared with us lots of stories of people presenting to the ED, and that’s the first time that things are found, and they get flown out and have to have valve replacements, have to have interventions at the time of diagnosis. So, we realized there was a much larger need than us just trying to figure out the prevalence of rheumatic heart disease there; we pivoted and said, “All right, well, let’s figure out how we can improve your access to echocardiography,” and so that is where this came from.
We totally shifted gears, so we used the techniques and things we were familiar with from our work in Uganda and other low and middle-income countries. These countries could be more well-resourced when it comes to cardiac specialty care. So, we decided to use that and see how it would fare, so that is how this came to be.
We implemented task shifting to train providers who need experience with echocardiography. This approach, successfully applied in other regions, addressed the long wait times and travel distances for echocardiograms. By integrating screening echoes into clinic visits, we aimed to provide quicker access to crucial information about patients’ heart health. This strategy allowed us to understand patients’ conditions better and more promptly, bypassing the delays associated with traditional echocardiogram processes.
So, that was the circuitous road to getting there.
Challenges in Accessing Cardiac Care for American Indian Populations
Luke Baldwin: Wow! I have so many questions now. You’ve touched on some barriers American Indians face in getting an echo, and I’m wondering about the hurdles cardiologists face in getting the results. Can you elaborate on these barriers? You’ve already mentioned transportation issues, but what about the availability of cardiac sonographers?
Sarah de Loizaga, MD: I think so, and you know, we’re saying American Indian very broadly here. But I would caution because we cannot generalize to say that for this specific population, the circumstances are the same as, you know, for those residing on Tribal lands in Oklahoma or Montana or California, whatever it may be, it’s very different for some of these remote populations. So, you know, that’s what we encountered with Ryan, where he was, there is a lack of cardiac specialty care, and access to getting those echocardiograms done. If you need some sort of intervention or something more specific, that is also challenging.
So, transportation is a barrier in this specific circumstance, but this might be a very different situation for those residing in a more urban location; ; so yes, I mean, I think there’s transportation that goes into this. I think there are many factors that some American Indian populations face, such as inadequate housing, insufficient phone access, not having specialty care available, and high rates of poverty, sometimes high use of substance abuse that also predisposes you to have various cardiomyopathies.
So, I think there are a lot of different barriers and they also vary from person to person. As you said, some are physical barriers, and others are not. I mean, even having culturally sensitive care is only sometimes something that is there. And so, I think all of those things can be challenges that are there for American Indian populations to be able to be integrated into care and remain in care.
Task Shifting in Healthcare: Empowering Non-Specialists
Luke Baldwin: So, I have a question about task shifting. Can you help me understand what that means and what the goal is?
Sarah de Loizaga, MD: In the US, the people who generally do an echocardiogram are sonographers trained in that, or cardiology-trained providers.
Task shifting [in this context] is taking the task of getting an echocardiogram and shifting it to people who wouldn’t usually do it. In our case, we trained a range of people, including providers who included MDs, but they were mainly internal medicine doctors. We also trained nurse practitioners and medical assistants, and we had one pharmacist who was also part of our group.
Only one of the people that we trained out of the 12 had any prior experience at all with echocardiograms. So, we trained them to obtain these images and do these screening echoes, which is not a full protocol. It does not replace a full echocardiogram, but it can teach them to get the images and information they need, at least some preliminary information, mainly focused on cardiac function and some valvular disease with a focus on the mitral valve and the aortic valve, and then trying also to pick up some pulmonary hypertension potentially.
Unlocking the Potential of Echocardiogram Screenings
Luke Baldwin: Maybe now’s a good time for you to provide a summary of this paper and this first study, and then we can dig into the details from there.
Sarah de Loizaga, MD: Essentially, we used task shifting to train 12 providers within IHS (Indian Health Service) to conduct these screening echos, with the idea that this would be integrated into the primary care clinic.
We found that we could teach the providers in this program how to conduct the echos. However, what we found challenging was getting this integrated into the clinic. They are incredibly busy. They said, “When I have a full patient panel with 20-minute slots, it becomes very challenging to integrate screening echos into that model.” So, that became the limitation of having full uptake of this, the way that we hoped to do it.
We tried to shift when we realized this, and we established an echo clinic, essentially, that they would staff on Fridays. They would slot people in if they identified individuals for whom they wanted an echo, or if people said yes, they’d like to have a screening echo, they would then be scheduled into those Friday clinics.
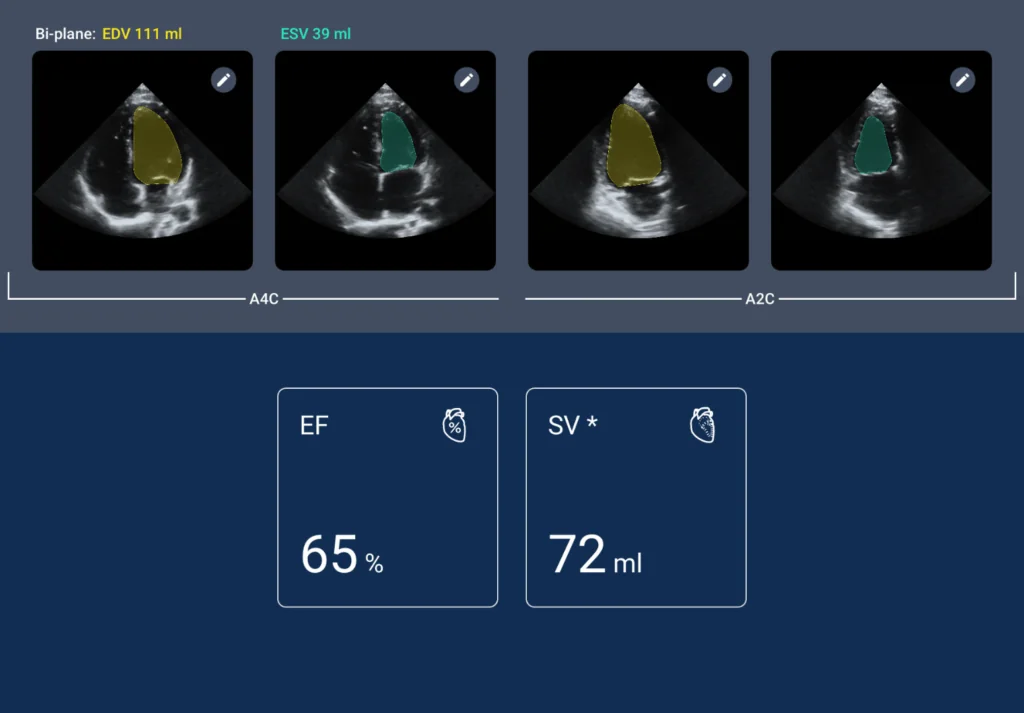
Though it didn’t have the uptake in the clinic we hoped for, we still screened over 600 patients. 14% of those had heart disease, with some of those knowing they had disease. However, 6% of those patients that we screened, had a new finding of heart disease that they previously had no idea about and also had no symptoms. So, you know, recognizing that there are patients out there who have no idea and would not have been captured otherwise without a screening echo.
Luke Baldwin: Wow. So, the screening echo was enough to pick up. Was it enough to actually identify? Was the goal to determine whether or not there was disease present, and then it would be confirmed with a full echo?
Sarah de Loizaga, MD: Yeah. So, we didn’t consider that diagnostic. These were considered screening. Then, anyone concerned about their screening echo was referred for a complete echocardiogram. That would be performed to get the full diagnostics. Recognizing this is an amazing opportunity helps bridge that gap, but it doesn’t replace having that full study to diagnose and connect them with care. So, that’s how we set up the program.
Luke Baldwin: It’s interesting because there are many conversations around this model or idea. People are aware that there may be more instances of disease in the population than we know. One reason is that it’s asymptomatic, so there’s no cause for concern. Other reasons include logistical barriers like distance to the clinic, lack of transportation, or limited space for testing. Do we justify a full echo? There’s talk about whether less trained healthcare providers can conduct exams that are informative enough for screening. Can they determine if someone needs a full echo or not? Would you say that there’s evidence to suggest that you’ve proven that?
Sarah de Loizaga, MD: No, I wouldn’t say we’ve proven that. In our study, all images were reviewed by cardiologists, which may limit scalability and sustainability. However, AI could play a significant role in interpretation, which we’re exploring further. Our current iteration incorporates AI to assist with interpretation but doesn’t replace human oversight. If AI flags abnormalities, we still recommend follow-up with a full echocardiogram and connection with a cardiologist.
We anticipate significant developments as we gather more data, particularly from remote areas with limited access to specialty care. While our study alone may not prove everything, there’s a promising correlation between the interpretations of trained providers and cardiologists, especially regarding ventricular size and function. With ongoing training and certifications, providers can improve their ability to interpret images, suggesting potential for future advancements.
Luke Baldwin: Yeah, that’s great. You mentioned how AI could assist in this, and I agree that further research is crucial. For the past 15 years, I have focused on point-of-care ultrasound, where training has always been limited to this area. The main hurdle to widespread adoption is the need for adequate training. Users must be proficient in operating the system, including interpretation. However, overcoming this learning curve is challenging. Is there a way to make training more accessible?
We’ve tried to address this issue at different companies I’ve worked for, but it’s complex. AI could play a significant role here, aiding in training and specialization. This could significantly boost confidence among users, making it easier to start imaging and gradually improve over time. Starting can be overwhelming and frustrating for new users who feel lost. AI could provide guidance, making the process less daunting.
Sarah de Loizaga, MD: Yeah, I agree. Auto-labeling was well-received. Sometimes, when you put the probe on, you’re unsure what you’re looking at. Having it labeled instantly is reassuring. It’s like, “Okay, great, I found it.” Even if I didn’t recognize it at first, I know what it is now that it’s labeled. It was a valuable tool that attracted people, and I believe it was instrumental.
Luke Baldwin: So, you mentioned that one of the questions you answered was, can these healthcare professionals be trained to use ultrasound, and you were able to show that through this work. What did your training protocol look like? How intensive was it?
Sarah de Loizaga, MD: Yeah. We started with online modules via GUSI (The Global Ultrasound Institute) and a two-day hands-on session. We conducted didactic ultrasound sessions during this session, teaching them various views and techniques. They also had the chance to practice with mentors guiding them. We provided volunteer models for practice over two days.
Afterward, we had four weeks of mentored scanning, where experts integrated into the clinic setting. They accompanied providers during patient scans, offering immediate feedback and guidance. A transition to independent scanning followed this intensive training period.
Reflecting on the process, we found it effective. In the future, we aim to incorporate more follow-up, such as group image reviews and ongoing feedback. Despite initial doubts, even participants with minimal medical background produced impressive images post-training. It was truly gratifying to witness their progress.
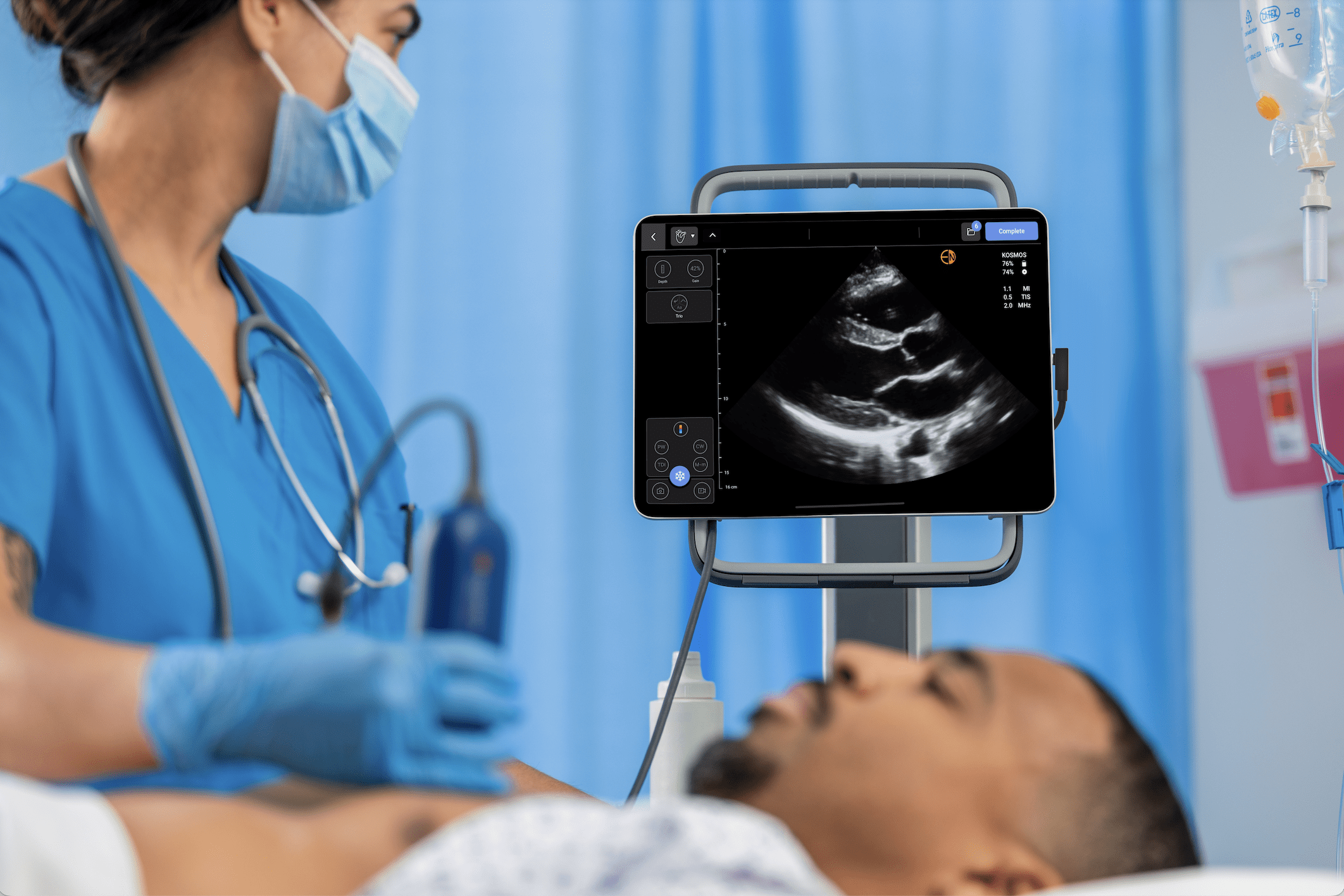
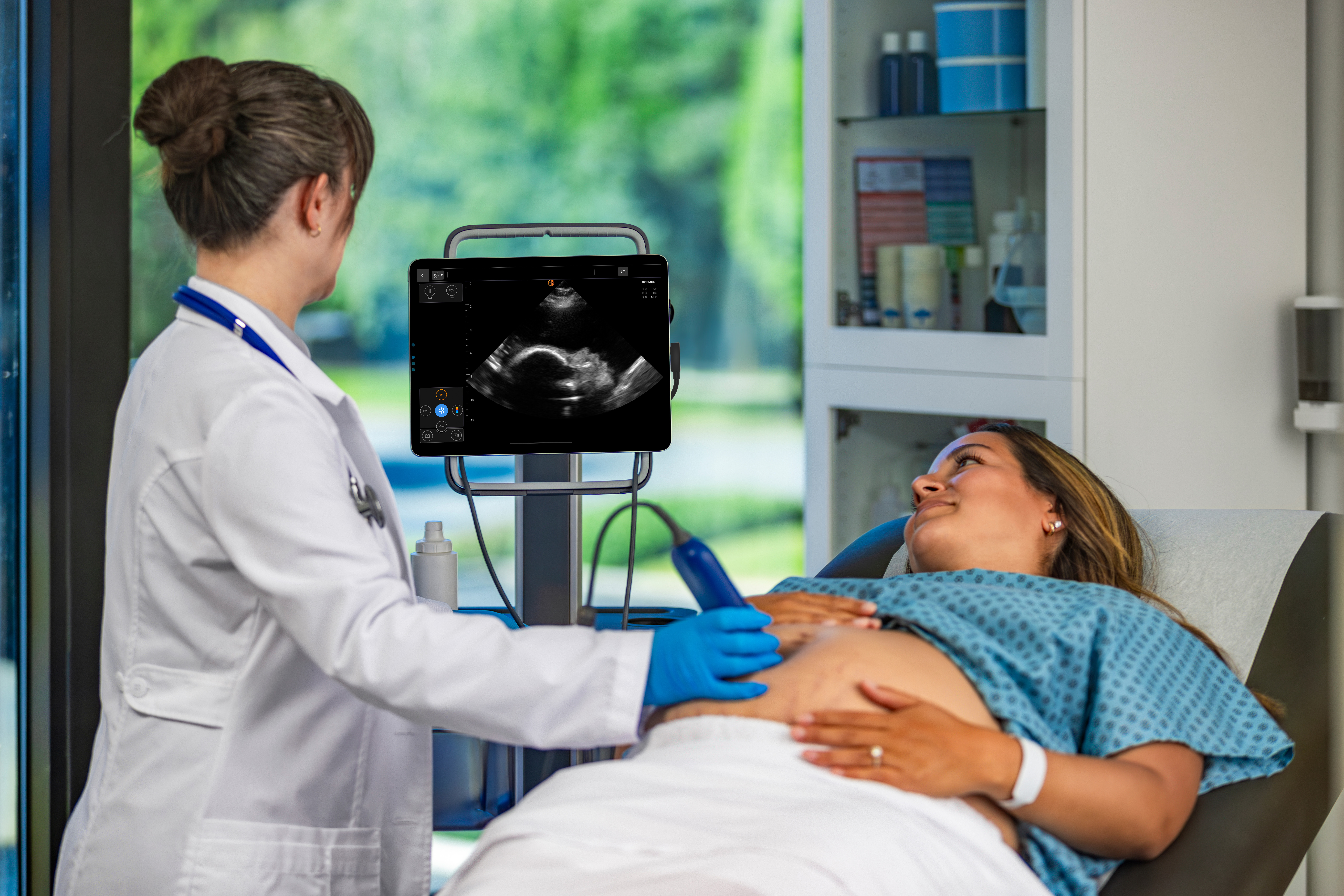
Choosing the Kosmos Ultrasound System: A Strategic Decision
Luke Baldwin: That’s great. So, let’s talk a little bit about that. You are working with our product, the Kosmos ultrasound system. It’s not a cart-based echo machine; it’s squarely within the point-of-care ultrasound space. What are some advantages, or why did you choose that direction instead of a laptop system? You know there are lots of other systems out there. What was it about the Kosmos that stood up?
Sarah de Loizaga, MD: Yeah. The AI features that come with it, the navigation of the auto labeling, and the big one for us, both the Continuous Wave and Pulsed Wave Doppler. So, we looked at valvular disease, which we wanted to have in our protocol. Knowing and talking with Ryan about the kind of pathology he sees and is most concerned about was also a huge benefit. And so, all of that together made it a perfect fit for those projects.
Community Partnership for Sustainable Solutions
Luke Baldwin: Thinking of the research you’ve done so far, are there any cases or anecdotes that stood out to you? Any unexpected learnings? Related to your passion for addressing healthcare disparities, were there any “Aha moments” or sparks in working with professionals and testing ideas to mitigate disparities?
Sarah de Loizaga, MD: One of the most rewarding and exciting aspects of my job was screening patients and gathering their feedback. Their overwhelmingly positive responses were truly heartening, and I was grateful for the opportunity to ease their burden compared to their usual experiences.
Realizing the impact of our work was rewarding. Although some aspects, like integrating into clinics, didn’t progress as expected, it prompted us to reconsider our approach. Discovering that nearly 6% of patients were unaware of their conditions reinforced our belief in the effectiveness of our methods.
This realization fueled our determination to refine our approach. Knowing we can potentially prevent emergency room visits, emergent procedures, and costly transfers by identifying conditions earlier is incredibly motivating. Our approach is effective; we must adapt and improve our methods.
Sarah de Loizaga, MD: Yeah. The AI features that come with it, the navigation of the auto labeling, and the big one for us, both the Continuous Wave and Pulsed Wave Doppler. So, we looked at valvular disease, which we wanted to have in our protocol. Knowing and talking with Ryan about the kind of pathology he sees and is most concerned about was also a huge benefit. And so, all of that together made it a perfect fit for those projects.
Research Implications and Future Directions
Luke Baldwin: What are the implications of this research, and what’s next? What does the future hold, and what areas still need exploration?
Sarah de Loizaga, MD: What we are currently doing is that we really have reimagined this—and that didn’t change anything about the task shifting or using Kosmos; that is still happening because that worked. But now, we are partnering with the tribe through their community health representative program.
I think I mentioned they are like community health workers, and they have a unique relationship with the community. There’s a lot of trust there, so I think one of the things that we did run into is that some people are just wary of anything that says research, because there have been a lot of injustices in the past. So, some people are very reluctant to be involved in anything that requires research. So, it’s exciting to see where we go with partnering with the tribal CHR program and that there is a trusted relationship.
We reimagined this and used a community-based participatory research approach this time. So, with this new work that we’re doing, the entire first year is about partnering with the community and co-developing the program. So, we can bring our expertise, we know how to task shift, we know how to teach people to get these screening echoes, we can bring that to the table. They Know what will and will not work in their community, so they bring that expertise. Then, together, we can partner to design this new program to address structural heart disease within their community to have early detection of heart disease, connection to care, and support wrap-around services to keep people in care.
So, I think that approach has been incredible to see as it’s developed over this past year, and the ideas that the community brought to the table about how this will actually work, and so I’m excited that we’re going to be able to move this forward with the community really helping to design it.
Luke Baldwin: It’s exciting. I’ve lost count, but many different people are working to solve similar problems. Sometimes it’s rural medicine, sometimes it’s modern metro areas that are trying to solve a similar issue in terms of access to cardiac screenings and whatnot. What advice might you have for someone who’s got their sights on solving a similar problem, as they’re thinking about doing research or tackling this? What would you say to them?
Sarah de Loizaga, MD: I may be a convert. One of our co-investigators focuses on community-based participatory research, and I am drawn to its potential to revolutionize our approach. While it’s an investment, partnering with the community for the first year allows us to tailor our approach to their needs. Every community is unique, so ensuring our efforts have the greatest impact is crucial. By listening to their feedback and truly collaborating, we can increase buy-in and make our approach work for them. I’m very excited about this aspect; it is an excellent place to start.
Luke Baldwin: Yeah, certainly, buy-in is going to be key. You’ve developed a model for the training and education that some people need.
You mentioned that once you opened up your clinic, you’re able to do 600 screenings, and that before, it would take months to get results. So, what was it like before you opened up your clinic? How many screenings were being done?
Sarah de Loizaga, MD: Well, they didn’t do echo screenings at all. So, the way that you would come into the system would be the patient has a complaint, like they are saying, “I feel short of breath every time I walk up the stairs,” or the provider had to have a concern. So, they came in for an exam, and they heard a murmur, so there was no echo screening being done that they offered. Something had to raise a flag to refer them for an echo.
Luke Baldwin: So, they were just too late when they came in?
Sarah de Loizaga, MD: I mean, only for some. Some people were caught early enough if they interacted with the Indian Health Service and got to the clinic appointment for their diabetes or hypertension or whatever it may be. But Ryan definitely saw multiple cases and instances of people who didn’t come to medical attention and had heart issues identified only when they showed up in the emergency department, which is not the ideal place to find out that you have major issues going on with your heart.
Luke Baldwin: So, following up on that, you found 6% of your population had undiagnosed structural heart disease, which could be extrapolated to like 100,000 people in that community nationwide; so, what happens with those patients after they have that diagnosis?
Sarah de Loizaga, MD: Yeah. We’re still sorting through this data, so stay tuned. But they go back and enter the system, where they get a referral to have an actual complete echocardiogram done, and then they connect with cardiac care.
I’m sure you’re anticipating the issue we’re trying to address with this new approach: the system is flawed. So, it’s very challenging. Between the tribal community health representatives, and IHS, no one talks to the cardiac specialists, and none of the systems are integrated, so it’s very inefficient and set up to fail easily.
You know, a year-long process that we’ve been doing now with the community is realizing that they need, and want, a case manager. So, when they screen positive, you connect with the case manager who’s dedicated to patients who have screened positive for heart disease and can help bridge the gaps between the various services. A positive screen needs to be referred to IHS, IHS needs to approve the referral, which must then go to the cardiologist. The patient then has to call. It’s just one thing after another. So that’s one thing we will be testing in this new approach that the community really said we need.
Luke Baldwin: Another thing that stood out to me in the survey responses for people who elected not to get the echo screen was that 22% wanted to avoid seeing a problem or to find out if they had it.
Sarah de Loizaga, MD: Yeah, that is one thing that also surprised me. It was the belief that if you got an echo, even if your heart was fine, it was like you were asking for something to be wrong with it, which I was just like, “Wow. Okay.This is not even an access issue; it works within those cultural beliefs, but it’s really interesting.” I would never have realized that unless we had done those surveys and asked patients because, in my mind, it’s like “screening to find something early, yes, please, whatever it is, I’ll do it if it helps me to stay healthy. “ Yeah, it was very interesting to hear that.
Seeing that our new program is a multi-pronged approach to the whole patient journey will be interesting. So, it’s from awareness all the way through to treatment. The way that we envision it is you have awareness, you have screening, you have confirmation, and then you have the treatment and follow-up. So, it’ll be really interesting to see what we do around awareness and the partnership with the community health representatives.
We created a Community Research Leadership Board that has been phenomenal. I wonder if partnering with the tribe and building awareness around this may help alleviate some concerns about asking for trouble if you do a screening echo. So, it’ll be interesting to see in this new study.
Luke Baldwin: I love hearing about your process and am a big fan of your work. It’s fascinating how your story evolved from initially looking at strep cases to exploring the prevalence of rheumatic heart disease and beyond. Your approach now seems much more holistic and comprehensive, which is impressive. The process is very similar to the design process at EchoNous when developing a new product or anything —you start with an idea and then uncover its broader implications. What’s great about your team is your perseverance. Instead of backing off when things get complex, you dig deeper to address the issue thoroughly. That’s truly encouraging.
Sarah de Loizaga, MD: Thank you. Yeah.
Luke Baldwin: It’s been an absolute pleasure chatting with you. I always find these conversations insightful. As a company, we’re dealing with a wide range of use cases and user types, which makes each discussion like this one valuable. Exploring specific use cases provides a deeper understanding, almost like a microcosm of broader challenges. If our solution can excel in this setting with its unique population and objectives, it will spark ideas for further innovation and development across different scenarios.
Sarah de Loizaga, MD: Chris Longenecker heads up this Health Equity Research Network. It’s a part of his premise behind reciprocal innovation. And so, it’s like, how can you learn from these different environments? From low and middle-income countries, for example, we’re talking about, and you use it in American Indian reservation. How do those things translate back and forth so that we can learn from each other? So that’s what you’re talking about.
Luke Baldwin: I just spoke with Ram Fish from 19 Labs, one of our partners. They’ve developed a telemedicine platform integrated into an Android system, offering various diagnostic tools like pulse ox and glucose monitoring, among others. We’ve also integrated our ultrasound system into their platform. During our conversation, Ram mentioned how developing rural communities find innovative ways to bypass traditional communication barriers, much like how cell phones replaced landlines. Developing these new care models could potentially surpass outdated healthcare systems, providing better access to healthcare. It’s an intriguing concept to consider, especially with the immediate access to answers and consultations, regardless of proximity to a doctor. It’s an interesting idea.
Sarah de Loizaga, MD: Yeah, it’s exciting times ahead. There’s a lot of new things on the horizon.
Luke Baldwin: Awesome. We appreciate you and thank you so much for your time.
To learn more about Kosmos, contact us today!
To read Dr. de Loizaga’s recent article, “Deployment of Point-of-Care Echocardiography to Improve Cardiac Diagnostic Access Among American Indians.” Click here!